Professor Emeritus
Academic History
- B.Sc. Biology (1981), Univ. Zagreb;
- M.Sc. Cell Biology (1982), Univ. Zagreb;
- Ph.D. Botany (1988) Michigan State Univ.;
- Postdoctoral Fellow (1988-89), Univ. Saskatchewan;
- Research associate (1989-91) and Research officer (1991-93) NRCC Plant Biotech. Institute, Sask.
Contact Information
- kunst@mail.ubc.ca
- 604-822-2351
- Office: Office Room 3207, Biological Sciences Building
My Links
Research Interests
Fatty acids and lipids are essential components of plant cells with diverse structural and signaling functions. They also form waxy cuticles on the plant surface required for plant protection against water loss, pathogens and insects, and serve as storage reserves in the seed that are exploited for human nutrition, used as industrial feedstocks, lubricants, and fuel. Research in my lab is directed toward understanding the following aspects of plant fatty acid and lipid metabolism:
1. Biosynthesis and secretion of cuticular wax. The cuticle is a thin hydrophobic layer which covers the outermost surface of the primary aerial tissues of land plants. It protects plants from uncontrolled water loss, UV radiation, as well as bacterial and fungal pathogens. It also mediates a variety of plant interactions with insects and prevents fusions between plant organs during development. The cuticle is synthesized by epidermal cells and its framework is provided by cutin, insoluble plant-specific polyester composed of C16 and C18 hydroxy and epoxy fatty acids and glycerol. The cutin matrix is embedded in and overlaid with cuticular waxes, complex mixtures of mostly very-long-chain fatty acid (VLCFA) derivatives easily extractable by organic solvents.
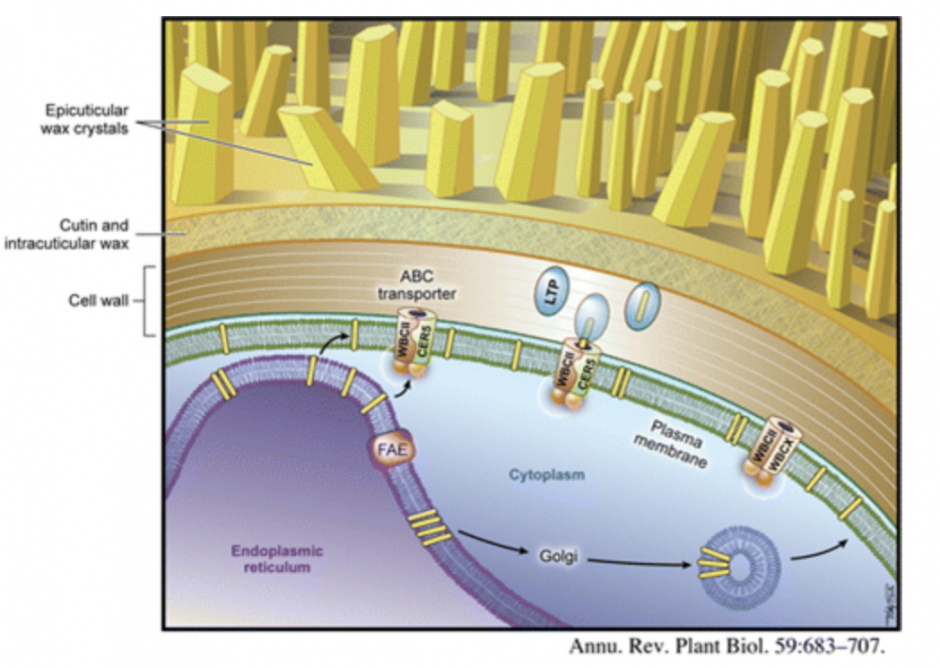
Similar wax constituents comprising cuticular wax of most plant species suggest that the basic mechanisms involved in wax production and transport to the cuticle are highly conserved in the plant kingdom, but our understanding of these processes is limited. We are using wax-deficient eceriferum mutants, standard reverse genetic techniques, as well as genomic chemical and molecular cell approaches in Arabidopsis thaliana to:
• identify and functionally characterize gene products involved in wax biosynthesis and secretion
• gain insights into regulation of wax deposition in epidermal cells
• establish subcellular localization of the enzymes of wax biosynthesis, and
• determine the mechanism of wax transport to the cuticle
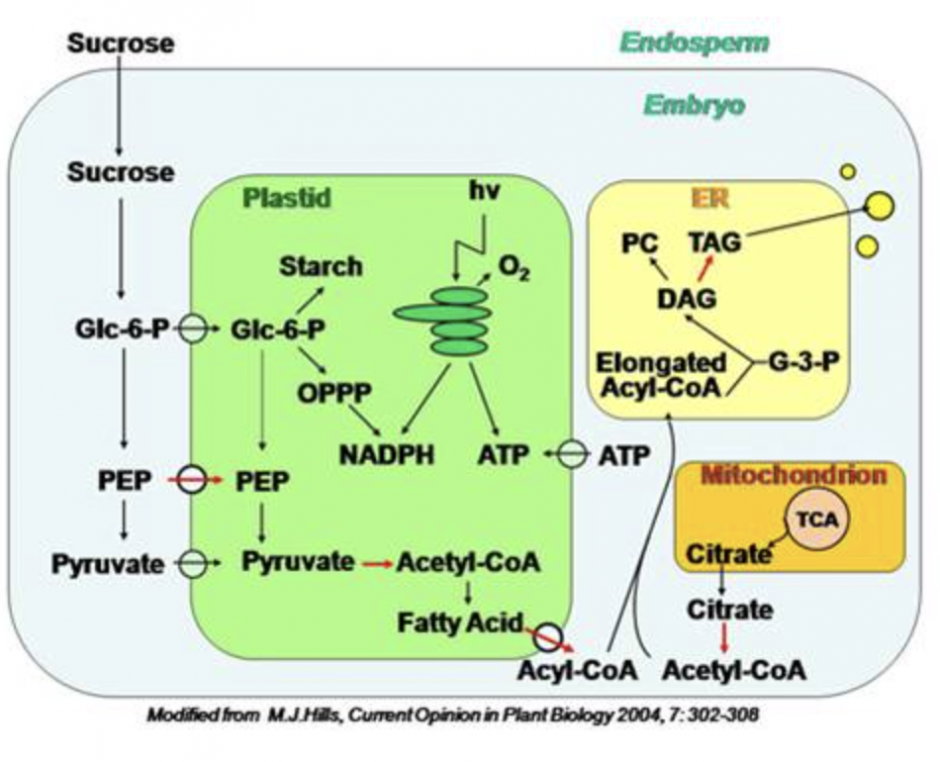
2. Regulation of seed storage oil production. As the pathways of oil biosynthesis in seeds are becoming better understood it is clear that total seed oil content can be altered by the manipulation of transcription factors that control plant oil biosynthesis, or manipulation of specific enzymes involved in this process. Oil biosynthetic enzymes can be broadly divided into two categories: those controlling fatty acid biosynthesis (source), and those involved in storage triacylglycerol (TAG) assembly (sink). This project is aimed at identifying key regulators and enzymes determining the seed oil content in Arabidopsis thaliana and their relative contributions to the overall levels of oil accumulation.
Selected Publications
P. Lam, L. Zhao, N. Eveleigh, Y. Yu, X. Chen, and L. Kunst (2014) The exosome and trans-acting siRNAs regulate cuticular wax biosynthesis during Arabidopsis inflorescence stem development. Plant Physiol. Dec. 11: [Epub ahead of print].
C. Nawrath, L. Schreiber, R. Franke, N. Geldner, J. J. Reina-Pinto and L. Kunst (2013) Apoplastic Diffusion Barriers in Arabidopsis thaliana. The Arabidopsis Book 2013 (http://www.bioone.org/doi/pdf/10.1199/tab.0167)
T. Roscoe and L. Kunst (2013) Seed Power: Increasing Oil Content by Redirecting Carbon Flux During Development. International Innovation Article 2013
T. M. Haslam and L. Kunst (2013) Extending the story of very-long-chain fatty acid elongation. Plant Science 210: 93– 107.
T. M. Haslam and L. Kunst (2013) Wax Analysis of Stem and Rosette Leaves in Arabidopsis thaliana. Bio-Protocols. (http://www.bio-protocol.org/wenzhang.aspx?id=782)
E. Sakuradani, L. Zhao, T. M. Haslam and L. Kunst (2012) The CER22 gene required for the synthesis of cuticular wax alkanes in Arabidopsis thaliana is allelic to CER1. Planta 237:731–738.
T.M. Haslam, A. Mañas Fernández, L. Zhao, and L. Kunst (2012) Arabidopsis ECERIFERUM2 is a component of the fatty acid elongation machinery required for fatty acid extension to exceptional lengths. Plant Physiol. 160: 1164-1174.
P. Lam, L. Zhao, H.E. McFarlane, M. Aiga, V. Lam, T.S. Hooker, and L. Kunst (2012) RDR1 and SGS3, components of RNA-mediated gene silencing, are required for regulation of cuticular wax biosynthesis in developing stems of Arabidopsis. Plant Physiol. 159: 1385-1395.
Shi, L., Katavic, V., Yu, Y., Kunst, L. and Haughn, G. (2012) Arabidopsis glabra2 mutant seeds deficient in mucilage biosynthesis produce more oil. Plant J. 69, 37-46.
L. Zhao, V. Katavic, F. Li, G. W. Haughn, and L. Kunst (2010) Insertional mutant analysis reveals that LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis. Plant J. 64, 1048-1058.
L. Kunst and L. Samuels (2009) Plant cuticles shine: advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 12: 721-727
X. Wu, F. Beaudoin, F. Li, R. P. Haslam, J. E. Markham, H. Zheng, J. A. Napier and L. Kunst (2009) Functional characterization of the Arabidopsis thaliana β-ketoacyl-CoA reductase candidates of the fatty acid elongase. Plant Physiology 150:1174-1191.
A. DeBono, T. Yeats, J.K.C. Rose, D. Bird, R. Jetter, L. Kunst and A.L.Samuels (2009) LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell 21: 1230-1238.
F. Li, X. Wu, P. Lam, D. Bird, H. Zheng, L. Samuels, R. Jetter and L. Kunst (2008) Identification of the wax ester synthase/acyl-coenzyme A:diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol. 148:97-107.
R. Jetter and L. Kunst (2008) Plant surface lipid biosynthetic pathways and their utility for metabolic engineering of waxes and hydrocarbon biofuels. Plant J. 54: 670–683.
L. Samuels, L. Kunst, and R. Jetter (2008) Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 59: 683-707.
S. Greer, M. Wen, D. Bird, X. Wu, L. Samuels, L. Kunst, and R. Jetter (2007) The cytochrome P450 enzyme CYP96A15 is the mid-chain alkane hydroxylase responsible for formation of secondary alcohols and ketones in stem cuticular wax of Arabidopsis thaliana. Plant Physiol. 145: 653-667.
O. Rowland, R. Lee, R. Franke, L. Schreiber and L. Kunst (2007) The CER3 gene from Arabidopsis thaliana is allelic to WAX2/YRE/FLP1 and is required for cuticular wax biosynthesis. FEBS Lett. 581: 3538–3544.
D. Bird, F. Beisson, A. Brigham, J. Shin, S. Greer, R. Jetter, L. Kunst, X. Wu, A. Yephremov, and L. Samuels (2007) Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J. 52: 485-498.
C. Lai, L. Kunst and R. Jetter (2007) Composition of alkyl esters in the cuticular wax on inflorescence stems ofArabidopsis thaliana cer mutants. Plant J. 50: 189-196.
T. S. Hooker, P. Lam, H. Zheng, and L. Kunst (2007) A core subunit of the RNA-processing/degrading exosome specifically influences cuticular wax biosynthesis in Arabidopsis. Plant Cell 19: 904–913.
O. Rowland, H. Zheng, S.R. Hepworth, P. Lam, R. Jetter, and L. Kunst (2006) CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol. 142: 866-877.
M.C. Suh, A. L. Samuels, R. Jetter, L. Kunst, M. Pollard, J. Ohlrogge, and F. Beisson (2005) Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol. 139: 1649–1665.
H. Zheng, O. Rowland, and L. Kunst (2005) Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell 17: 1467-1481.
H. Moon, G. Chowrira, O. Rowland, B. J.Blacklock,M. A. Smith, and L. Kunst (2004) A root-specific condensing enzyme from Lesquerella fendleri that elongates very-long-chain saturated fatty acids. Plant Mol. Biol. 56: 917-927.
J. A. Pighin, H. Zheng, L. J. Balakshin, I. P. Goodman, T. L. Western, R. Jetter, L. Kunst, and A. L. Samuels (2004) Plant cuticular lipid export requires an ABC transporter. Science 306: 702-704.